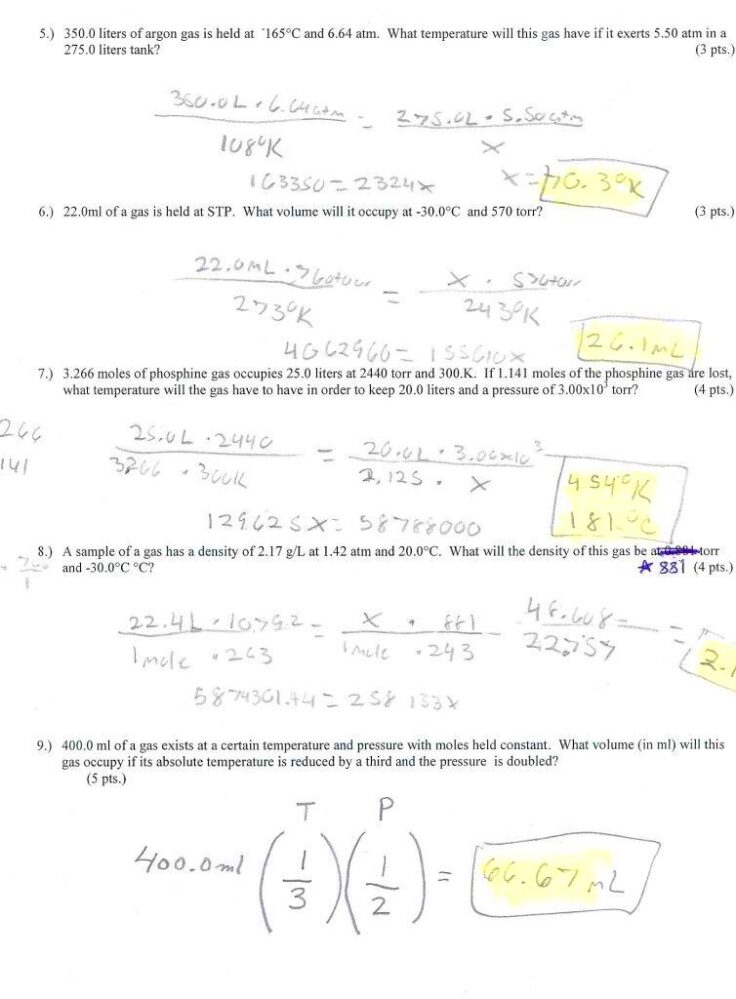
Charles’s Law Problems Worksheet with Answers: Understanding the Concepts
In chemistry, one of the things that people talk about is Charles’ law. This law explains how gases respond when the conditions are changed. It says that when pressure remains constant, the volume of a gas increases with temperature. This relationship has implications for many scientific fields including cooking and engineering. This article, therefore, aims at exploring not only the basic principles but also real-life applications as well as common issues associated with Charles’ Law. So let us begin!
Understanding the Basic Concepts of Charles’s Law

In order to understand Charles’s Law, one must first comprehend its fundamental aspects:
- Volume (V): The space that a gas occupies, usually measured in liters.
- Temperature (T): The degree of hotness or coldness, measured in Kelvin for gas law calculations.
- Pressure (P): The force that the gas exerts on the walls of its container, which remains constant in this law.
Charles’s law can be expressed mathematically as:
V1/T1 = V2/T2
The formula indicates that an increase in temperature (T) produces an increase in volume (V) together with constant pressure. This understanding allows predicting behavior of gases under different conditions.
Real-Life Applications of Charles’s Law

All over the world, Charles’s Law is applied every day in different ways. Here are a few examples:
- Hot Air Balloons: The air inside the balloon expands when heated, causing the balloon to rise. This is a direct application of Charles’s Law.
- Cooking: When boiling pasta, the steam produced expands, and understanding this helps chefs adjust cooking times and methods.
- Aerospace Engineering: Engineers consider gas laws when designing aircraft, ensuring they operate efficiently at different altitudes and temperatures.
- Weather Balloons: These balloons expand as they rise in the atmosphere, providing valuable data about temperature and pressure changes.
In general, Charles’ Law serves a critical function in numerous areas by improving our comprehension of how gases act under various circumstances.
Common Problems Involving Charles’s Law
Charles’s Law, amongst others, presents different types of problems that require an understanding of the interaction between temperature and volume. The following are some examples for consideration:
- Heating a Gas: If you heat a gas in a sealed container, what happens to its volume?
- Cooling a Gas: Conversely, if a gas cools, how does that affect its volume?
- Volume Change with Temperature: What if you know the initial and final temperatures? How do you find the new volume?
- Conversions between Units: Sometimes, you may need to convert temperatures from Celsius to Kelvin, which is crucial for calculations.
We can examine an uncomplicated illustration:
A balloon has a volume of 5 liters at 300 K. If the temperature increases to 600 K, what will the new volume be? This situation illustrates how Charles’s Law is applied in everyday problems.
Step-by-Step Guide to Solving Charles’s Law Problems
If you take these actions into consideration; solving Charles’s Law problems can be simple:
- Identify Known Values: Write down the initial volume (V1), initial temperature (T1), and the final temperature (T2).
- Convert Temperature: Ensure temperatures are in Kelvin. Use the formula: K = °C + 273.15.
- Apply the Formula: Use the Charles’s Law equation V1/T1 = V2/T2 to solve for the unknown.
- Calculate: Rearrange the formula as needed to isolate the variable you’re solving for.
- Check Units: Ensure that your final answer is in the correct units (usually liters).
When you take these steps systematically, you can solve any issue on Charles’s Law with confidence.
Practice Problems for Better Understanding
In dealing with profound comprehension of Charles’s Law, one can agree that practice is a necessity. The following are some of the practice problems which will help you in checking your understanding:
| Problem | Given Values | Find |
|---|---|---|
| 1 | V1 = 4 L, T1 = 300 K, T2 = 600 K | V2 = ? |
| 2 | V1 = 10 L, T1 = 273 K, T2 = 546 K | V2 = ? |
| 3 | V1 = 5 L, T1 = 320 K, T2 = 160 K | V2 = ? |
Dealing with listed approaches, tackle these puzzles. After working on them, validate your solutions:
- Problem 1: V2 = 8 L
- Problem 2: V2 = 20 L
- Problem 3: V2 = 2.5 L
If you keep practicing regularly, you will enhance your comprehension of Charles’s Law and its uses!
Frequently Asked Questions about Charles’s Law
Questions often arise about Charles’s Law, particularly if the concept is new to you. Some of the common queries raised are as above that might help eliminate ambiguity in your mind:
- What is the relationship between temperature and volume?The relationship is direct; as the temperature of a gas increases, its volume increases as well, provided the pressure remains constant.
- Why is temperature measured in Kelvin?Kelvin is used in gas law calculations because it avoids negative values, which simplifies the math and ensures that the relationships hold true.
- Can Charles’s Law be applied to liquids?No, Charles’s Law specifically applies to gases. Liquids have a relatively constant volume and do not expand in the same way as gases do.
- How can I remember Charles’s Law?A useful tip is to remember the phrase “volume up, temperature up,” which captures the essence of the law.
- What happens if pressure is not constant?If the pressure varies, you’ll need to use other gas laws, like the combined gas law, which takes into account pressure changes as well.
Bursts of questions related to Charles’s Law applications will assist in better grasping it. If you need any clarification, go ahead and research or ask someone who knows!
Conclusion on Charles’s Law and Its Importance
Charles’s Law is significant in the understanding of gas behavior; it finds practical applications in different areas like meteorology, engineering and ordinary life. By understanding the relationship between temperature and volume, you not only increase your scientific understanding but also develop problem solving abilities that can be used in real life. Whether you are planning an experiment or just taking a hot air balloon flight, the insights offered by Charles’s Law deepen your appreciation of nature’s governing principles.