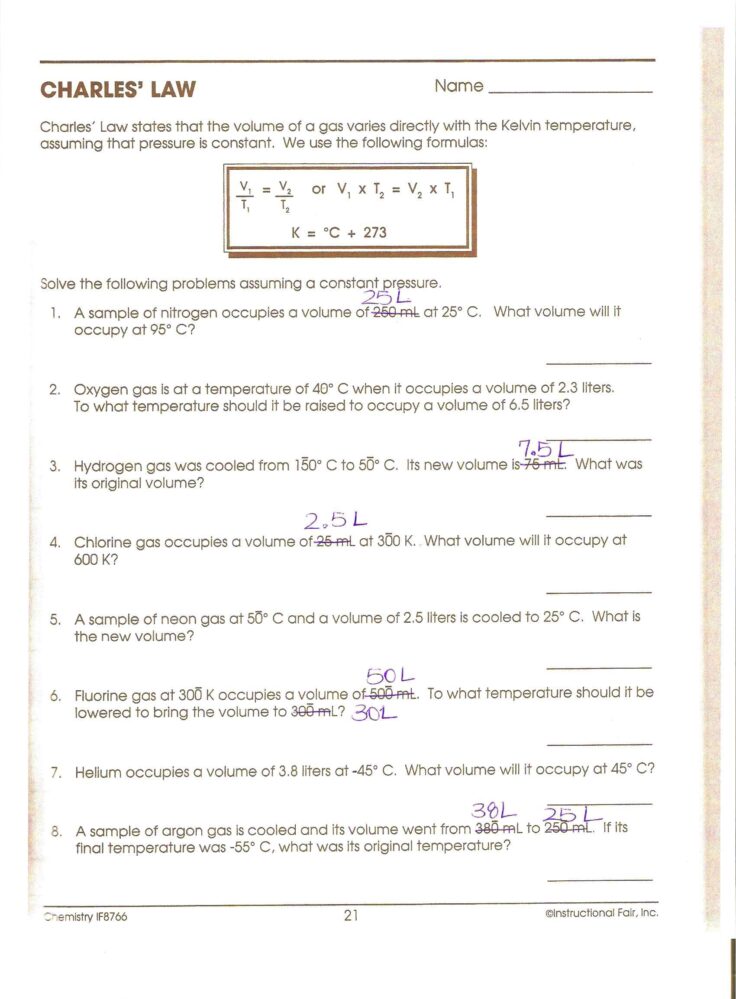
Charles’s Law Worksheet: Mastering the Basics
Charles’s Law is a principle in chemistry that once you understand it, makes you appreciate the elegant workings of the universe. Named after the French scientist Jacques Charles this law explains how the volume and temperature of a gas are related. It serves as a reminder of how science can clarify our perception of everyday occurrences. For instance it helps us understand why a balloon filled with air puffs up when exposed to heat. This concept connects our experiences with scientific principles.
Understanding the Basic Principles
Charles’s Law basically states that the volume of a gas increases with its temperature as long as the pressure stays the same. It may sound a bit scientific but let me explain it with a personal touch. Picture holding a balloon outside on a cold morning. It feels small and tight. Now if you take that balloon into a warm room you’ll see it puff up more. That’s Charles’s Law working. The air inside the balloon makes the gas molecules move speedily and spread out, making the balloons volume larger.
Charles Law can be mathematically represented as follows
- V1/T1 = V2/T2
Where:
- V1 is the initial volume
- T1 is the initial temperature
- V2 is the final volume
- T2 is the final temperature
This connection stays valid as long as the pressure stays the same, which is frequently the situation in various real world situations. Grasping this concept proves useful in a range of applications such, as creating hot air balloons or regulating the pressure within car tires.
Key Variables in Charles’s Law
When dealing with Charles’s Law there are several important factors to consider. These factors play a role in comprehending how the law is relevant to various scenarios.
- Volume (V): This is the space that the gas occupies. It’s crucial because, according to Charles’s Law, it changes with temperature. In practical terms, it’s like measuring the size of the balloon or the amount of space the gas fills in a container.
- Temperature (T): This is measured in Kelvin. Remember, you can’t use Celsius or Fahrenheit directly in Charles’s Law calculations. If you’re working with Celsius, you need to convert it to Kelvin first. The Kelvin scale starts at absolute zero, the point where gas molecules have minimal motion.
- Pressure (P): Although Charles’s Law assumes constant pressure, it’s worth noting that pressure and volume are related in other gas laws. Keeping pressure constant allows us to focus solely on the relationship between volume and temperature.
In our daily routines these factors constantly influence each other. Take heating a gas as an example like in a car tire. When you do the temperature rises and the gas volume may shift impacting the pressure. This interplay is a captivating showcase of nature’s principles that can be truly enlightening once grasped.
Formulating Charles’s Law Equations
Using Charles’s Law to come up with equations is similar to following a reliable recipe that helps you grasp how gases behave. The main formula V1/T1 = V2/T2 serves as a handy instrument for determining the impact of temperature on gas volume. Let me show you a practical way to apply this equation.
1. Recognize the Values Begin by jotting down the starting volume (V1), starting temperature (T1) and if you have it the ending volume (V2) or temperature (T2). Its similar to collecting your ingredients before preparing a meal.
2. Transform Temperatures into Kelvin: Keep in mind that the temperatures need to be expressed in Kelvin. To convert Celsius to Kelvin simply add 273.15 to the Celsius temperature. For example if the temperature is 25°C it would be equivalent to 298.15 K (25 + 273.15).
3. Adjust the Formula: Change the equation based on what you want to determine. For instance if you’re looking for the volume (V2) you would modify it like this.
- V2 = V1 × (T2/T1)
4. Insert the Numbers and Find the Solution: Replace the variables with the information you have in the adjusted formula. Its similar to blending components in the proportions to achieve the perfect flavor for your dish.
This approach makes it easier to grasp the impact of temperature changes on gas volume, be it for conducting a science experiment or just satisfying your curiosity about the scientific principles at play in everyday situations.
Solving Problems Using Charles’s Law
Tackling challenges using Charles’s Law is akin to piecing together a jigsaw puzzle where each fragment needs to align seamlessly. Allow me to walk you through the process in a manner.
1. Grasp the Issue: Begin by thoroughly examining the problem to discern the provided details and what you’re required to determine. As an illustration you could be informed that a balloon has a volume of liters at 300 K and be tasked with calculating its volume at 400 K.
2. If Needed Change Units Make sure all temperatures are expressed in Kelvin. If they aren’t convert them. This is important because Charles’s Law is applicable only with temperatures in Kelvin.
3. Utilize the Charles’s Law Equation: Implement the formula V1/T1 = V2/T2. Replace the variables with the values. In this case V1 is 2 L, T1 is 300 K and T2 is 400 K. Your task is to determine the value of V2.
- V2 = V1 × (T2/T1)
- V2 = 2 L × (400 K / 300 K) = 2.67 L
4. Review Your Result Take a moment to review your calculations for precision. An error in conversion or math can occasionally result in an incorrect outcome.
By following these steps you’ll discover that applying Charles’s Law to solve problems feels more natural making it easier to tackle even the most challenging questions.
Practical Applications of Charles’s Law
Charles Law is not merely an abstract idea; it finds practical use in enhancing our daily lives. Here are a few real world instances.
One of the most interesting uses of this concept is with hot air balloons. When the air inside a balloon gets heated it expands causing the balloon to go up. Pilots can manage how high or low the balloon goes by changing the air temperature based on Charles’s Law.
Air Conditioning Systems: The design of air conditioners involves the application of Charles’s Law. These systems regulate the air temperature to optimize volume and pressure for effective cooling.
Tires: Temperature fluctuations can impact the air pressure and volume in your vehicle tires. During winter the air inside the tires contracts making it crucial to regularly check tire pressure for safe driving.
4. Science Experiments: A lot of science experiments and shows use Charles’s Law to show how temperature and volume are connected. It’s a practical way to witness the principles of physics at work.
Common Items Even ordinary objects such as soda cans or inflatable playthings are influenced by the concepts of Charles’s Law. When the temperature shifts the gas volume within these items changes accordingly leading to potential expansion or contraction.
Grasping the relevance of Charles’s Law in these situations not only enhances your understanding of concepts but also allows you to recognize the real world significance of these principles in your daily routine.
Common Mistakes and How to Avoid Them
When dealing with Charles’s Law there are some common errors that can catch even the most attentive learners off guard. Let’s explore these traps and how to avoid them. Consider this a helpful resource to make your experience with gas laws more enjoyable.
1. Overlooking Temperature Measurements: A common mistake is neglecting to switch temperatures to Kelvin. Picture attempting to bake a cake at 200°C without converting it to Fahrenheit – it’s sure to lead to some mix ups. Keep in mind that for the law to function properly temperatures need to be expressed in Kelvin. Just add 273.15 to your Celsius figure.
2. Mixing Up Volume and Pressure: It can be tricky to remember how pressure impacts volume differently than temperature. Charles’s Law works with pressure held steady, so if there’s a change in pressure, you’re probably looking at a different gas law, such as Boyle’s Law. To prevent errors in calculations, it’s important to keep these concepts separate.
3. Measuring Mistakes: Accuracy is key! When it comes to measuring things like volume or temperature make sure your tools are properly calibrated and your measurements are spot on. Even a small mistake in measurement can cause variations in your outcomes. Stick to trustworthy equipment and verify your measurements to be sure.
4. Neglecting to Verify the Conditions: Charles’s Law is applicable when the pressure remains constant. If your experiment or situation involves varying pressure keep in mind that you may need to incorporate gas laws or take into account other factors that can impact the outcomes.
5. Neglecting Figures When it comes to calculations significant figures are essential for ensuring precision. To prevent rounding mistakes make sure to monitor significant figures consistently during your computations.
By staying mindful of these errors you can approach Charles’s Law with more assurance and precision transforming potential challenges into valuable lessons.
Additional Resources for Learning Charles’s Law
To enhance your grasp of Charles’s Law, you can find a wealth of resources to assist you. Here’s a selection of materials that could prove useful:
2. Video Lessons and Tutorials Websites such as Khan Academy, Coursera and YouTube provide visual demonstrations and guided lessons. These resources can be especially beneficial for individuals who grasp concepts better through visual aids.
3. Engaging Simulations: Platforms such as PhET Interactive Simulations provide online laboratories where you can explore Charles’s Law through experiments. This interactive approach allows you to witness the concepts at play firsthand.
4. Collaborative Learning Platforms Engaging in study groups or online forums such as Stack Exchange or the r/learnmath subreddit can offer valuable support. Sharing challenges with peers can introduce fresh viewpoints and problem solving approaches.
Exercise Questions Platforms that focus on chemistry practice questions can assist in strengthening your comprehension. They provide a range of problems with different levels of difficulty allowing you to assess your abilities and expertise.
Diving into these materials will not only deepen your understanding of Charless Law but also add an element of fun and excitement to the learning process.
Frequently Asked Questions
When delving into Charles’s Law you might encounter these common questions along with simple explanations to help clarify any misunderstandings.
1. What is Charles’s Law in simple terms?
Charles Law states that the amount of gas is directly related to its temperature as long as the pressure stays the same. In simpler terms when you heat up a gas its volume gets bigger and when you cool it down its volume gets smaller.
2. Why do we need to use Kelvin for temperature in Charles’s Law?
The Kelvin scale is preferred as it begins at zero, the point where molecular movement stops. This ensures precision and significance in calculations. On the other hand the Celsius and Fahrenheit scales include values that don’t align with the direct relationship employed in Charles’s Law.
3. How does Charles’s Law apply to real-life situations?
Charles’s Law is relevant in situations like balloons getting bigger when warmed up, gases in car tires expanding in hot weather and how hot air balloons operate. Grasping this principle allows us to recognize the impact of temperature variations on gases, in real life situations.
4. Can Charles’s Law be used if the pressure is not constant?
Actually Charles’s Law is based on the assumption of pressure. If there are changes in pressure you’ll have to take into account other gas laws like Boyle’s Law which focuses on the connection between pressure and volume.
5. What should I do if my calculations seem incorrect?
If your outcomes don align with what you expected take a moment to review your temperature units make sure your measurements are precise and confirm that you’re applying the formula. A tiny error in these aspects can sometimes result in discrepancies.
These frequently asked questions are here to help clarify any doubts you may have and simplify your learning process regarding Charles’s Law.
Conclusion
Charles’s Law may appear simple at first glance but it reveals an intriguing facet of gas behavior in response to temperature changes. Its principles find application in scenarios ranging from balloon inflation to tire pressure management. By grasping the fundamentals of this law acknowledging common misconceptions and leveraging the abundance of resources at your disposal you not only gain intellectual understanding but also practical wisdom that deepens your worldview. Approaching these concepts with curiosity and mindfulness will not only enrich your comprehension of chemistry but also foster an appreciation for the scientific principles underpinning everyday occurrences.